The eClinical solutions market is expanding rapidly as the life sciences industry increasingly adopts digital technologies to streamline clinical trials and enhance drug development processes. eClinical solutions encompass a wide range of software and services, including clinical data management systems (CDMS), electronic data capture (EDC), clinical trial management systems (CTMS), randomization and trial supply management (RTSM), and electronic patient-reported outcomes (ePRO). These solutions are designed to improve efficiency, ensure regulatory compliance, reduce costs, and enhance data accuracy across the clinical trial lifecycle. Growth in the market is fueled by the rising complexity of clinical trials, the demand for real-time data analytics, and the shift toward decentralized and virtual trials. Additionally, the integration of artificial intelligence (AI), cloud computing, and big data analytics is transforming clinical research by enabling faster decision-making and better patient engagement. Pharmaceutical and biotechnology companies, contract research organizations (CROs), and academic institutions are increasingly investing in eClinical platforms to accelerate drug development timelines.
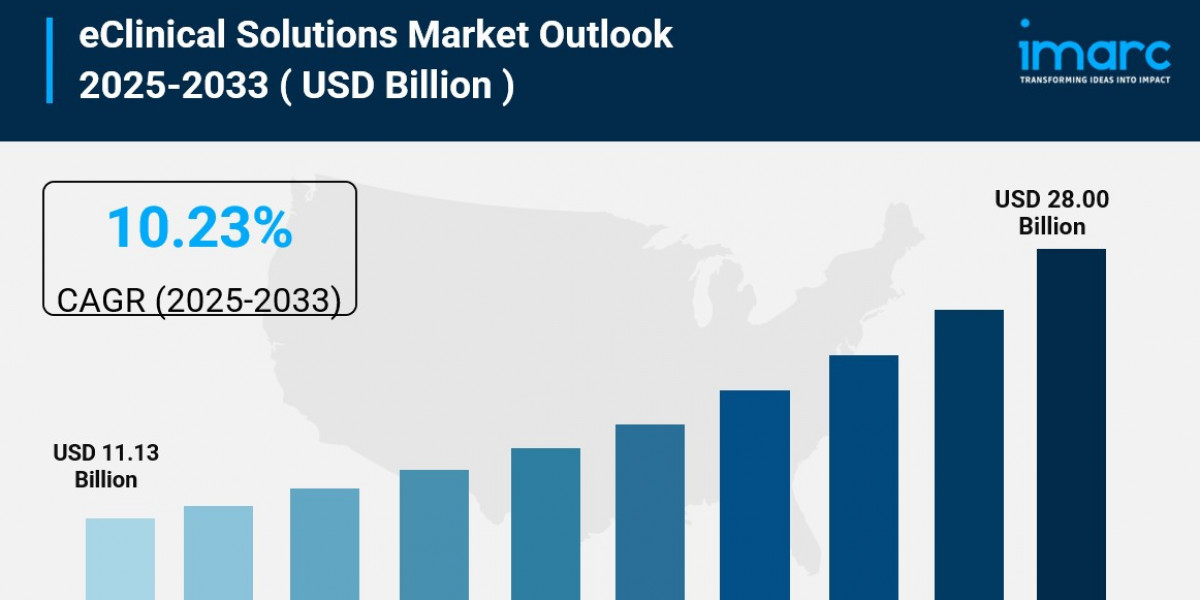
The global eClinical solutions market size reached US$ 10.1 Billion in 2023 and is expected to reach US$ 25.9 Billion by 2032, exhibiting a growth rate (CAGR) of 10.8% during 2024-2032. The extensive research and development activities conducted by biopharma and pharma companies, the increasing incorporation of software solutions in clinical trials, and the growing need for safety monitoring represent some key factors driving the market.
Key Trends
- Adoption of Cloud-Based Platforms: Cloud computing enables centralized data management, real-time collaboration, and secure storage, making it easier for organizations to manage multi-site clinical trials efficiently.
- Decentralized and Virtual Clinical Trials: The shift toward remote patient monitoring, telemedicine, and virtual trial models is driving demand for integrated eClinical solutions.
- Integration of Artificial Intelligence (AI) and Machine Learning (ML): AI/ML technologies are being leveraged for predictive analytics, patient recruitment optimization, risk-based monitoring, and faster decision-making.
- Data Standardization and Interoperability: Increasing emphasis on standardized data formats (e.g., CDISC) allows seamless integration of different clinical trial systems and facilitates regulatory submissions.
- Patient-Centric Approaches: eClinical solutions are incorporating patient-reported outcomes (ePRO) and wearable devices to enhance patient engagement, adherence, and overall trial quality.
Growth Drivers
- Increasing Complexity of Clinical Trials: Growing use of combination therapies, multi-center trials, and biologics requires efficient digital solutions to manage large volumes of data.
- Rising R&D Spending: Pharmaceutical and biotechnology companies are investing heavily in drug development, creating demand for streamlined clinical trial management.
- Regulatory Support: Favorable regulations and guidance encouraging the use of electronic systems and data capture technologies are driving market adoption.
- Need for Cost and Time Efficiency: eClinical solutions reduce manual errors, accelerate data collection, and improve trial timelines, leading to lower costs and faster go-to-market.
- Technological Advancements: Integration of cloud computing, AI, big data analytics, and wearable devices enhances operational efficiency and trial quality.
Request to Get the Sample Report: https://www.imarcgroup.com/eclinical-solutions-market/requestsample
Factors Affecting the Growth of the eClinical Solutions Industry:
Increasing Adoption of Digital Health Technologies:
The growing adoption of digital health technologies is significantly driving the growth of the eClinical solutions market. Pharmaceutical and biotechnology companies are increasingly leveraging advanced digital tools to streamline clinical trials, improve data management, and enhance overall operational efficiency. Innovations such as electronic data capture (EDC), electronic clinical outcome assessment (eCOA), and clinical trial management systems (CTMS) are being widely implemented to facilitate accurate, real-time data collection and analysis. The rising complexity of clinical trial protocols and the increasing volume of clinical data are further fueling demand for these solutions. Moreover, regulatory authorities are advocating for the use of digital technologies to ensure compliance, strengthen data integrity, and support more informed decision-making in clinical research, collectively boosting market growth.
Rising Demand for Personalized Medicine:
The growing focus on personalized medicine is another key driver of market expansion. Personalized medicine aims to tailor treatments to individual patient characteristics, which requires highly data-intensive and complex clinical trials. eClinical solutions provide the necessary technological infrastructure to manage and analyze these extensive datasets effectively. Advanced analytics and data integration capabilities offered by eClinical platforms enable researchers to evaluate patient-specific responses and identify potential side effects more efficiently. Additionally, the integration of genomic data and biomarker studies into clinical trials is increasing, necessitating sophisticated data management systems and further accelerating market adoption.
Growth in Outsourcing Clinical Trials:
The trend of outsourcing clinical trials to contract research organizations (CROs) is also contributing to market growth. Pharmaceutical companies are partnering with CROs to leverage their expertise, infrastructure, and technological capabilities in managing trials. CROs rely on eClinical solutions to provide comprehensive clinical trial management services, including data collection, monitoring, and analysis. The need to reduce operational costs, enhance trial efficiency, and accelerate time-to-market for new drugs is driving adoption. eClinical solutions allow CROs to manage multiple trials simultaneously while ensuring standardized processes, regulatory compliance, and scalability to meet the diverse demands of various sponsors.
Key Companies:
- Anju Software Inc.
- Clario
- Fountayn
- eClinical Solutions LLC
- eClinicalWorks
- Oracle Corporation
- PAREXEL International Corporation and Signant Health
eClinical Solutions Market Report Segmentation:
By Product:
- eCOA
- EDC and CDMS
- Clinical Data Integration Platforms
- Safety Solutions
- CTMS
- RTSM
- eTMF
- Clinical Analytics Platforms
CTMS represented the largest segment due to its critical role in streamlining clinical trial management and ensuring compliance with regulatory standards.
By Delivery Mode:
- Web-hosted (On-demand)
- Licensed Enterprise (On-premises)
- Cloud-based
Web-hosted (on-demand) accounted for the largest market share as it offers greater flexibility, scalability, and cost-effectiveness compared to traditional on-premise solutions.
By Development Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
Phase III represented the largest segment as it involves extensive testing on large patient populations, requiring significant data management and analysis.
By End Use:
- Hospitals
- Contract Research Organization
- Academic Institutes
- Pharma and Biotech Organizations
- Others
Contract research organization accounted for the largest market share due to the increasing trend of outsourcing clinical trials to specialized entities for efficiency and cost reduction.
Regional Insights:
- North America
- Asia-Pacific
- Europe
- Latin America
- Middle East and Africa
North America's dominance in the eClinical solutions market is attributed to advanced healthcare infrastructure, substantial research and development (R&D) investments, and favorable regulatory environments.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=4000&flag=C
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Also Read: https://youtu.be/QF4xxNyEnHM
Contact us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
United States: +1-201971-6302