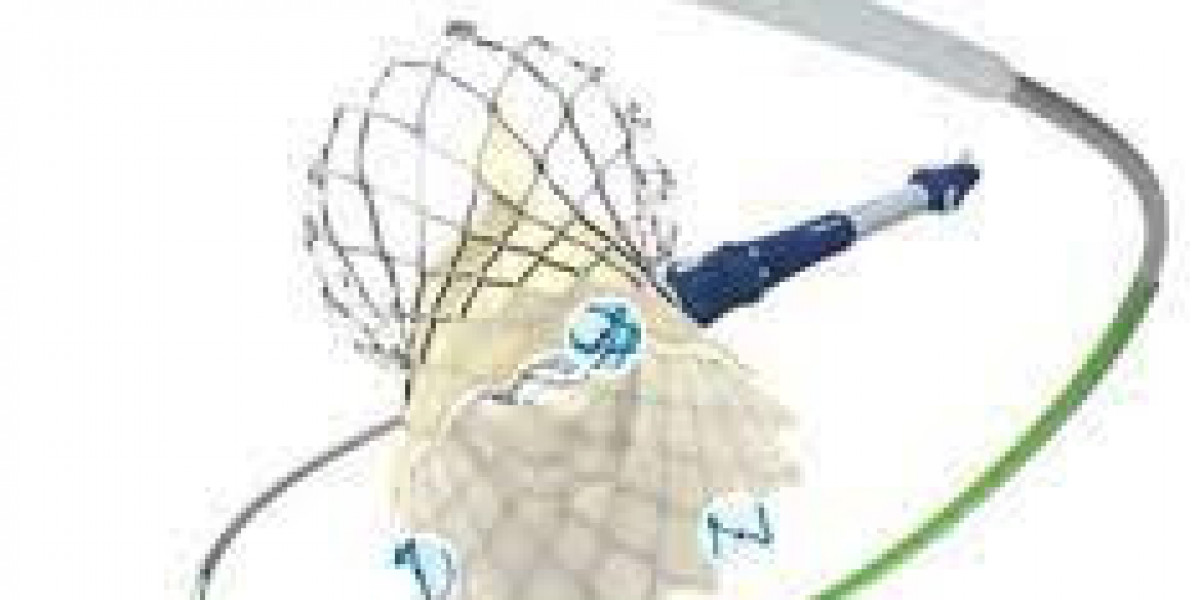
Heart valve devices market is heavily influenced by surgical outcomes, which vary according to device type and procedure method. Differences in mechanical, bioprosthetic, and transcatheter valve devices, combined with surgical technique selection, directly impact patient recovery, long-term device performance, and overall market adoption. Analyzing these outcomes allows healthcare providers, manufacturers, and policymakers to optimize treatment strategies and improve procedural efficiency, ensuring safer and more effective cardiovascular care.
Importance of Device Type in Surgical Outcomes
Device type plays a crucial role in determining procedural success. Mechanical valves offer long-term durability but require lifelong anticoagulation therapy, which carries a risk of bleeding complications.
Bioprosthetic valves reduce the need for anticoagulation but have limited durability, requiring potential re-intervention in the long term. Transcatheter valves, used in minimally invasive procedures, provide faster recovery but are suitable for specific patient populations. Selecting the appropriate device improves patient safety and enhances procedural outcomes, influencing the heart valve devices market.
Minimally Invasive vs. Open-Heart Procedures
Surgical outcomes are also affected by the procedure method. Open-heart surgeries remain standard for many complex valve replacements, offering long-term durability and suitability for diverse anatomical conditions.
Minimally invasive procedures, such as transcatheter aortic valve replacement (TAVR) and transcatheter mitral valve repair, offer reduced hospital stays, lower infection risks, and faster recovery. While these procedures are increasingly preferred for high-risk or elderly patients, procedural complexity and device compatibility must be carefully assessed to ensure optimal outcomes.
Role of Surgeon Expertise
Surgeon expertise significantly influences outcomes in heart valve procedures. Skilled surgeons reduce procedural errors, minimize complications, and ensure accurate device placement.
Training programs, continuous education, and experience with advanced devices enable surgeons to optimize procedural success. Hospitals with highly trained surgical teams see improved recovery rates, fewer re-interventions, and higher patient satisfaction, which encourages broader adoption of advanced heart valve devices.
Impact of Device Design on Patient Outcomes
Innovative valve designs have improved surgical outcomes across device types. Repositionable valves, self-expanding mechanisms, and hybrid materials reduce the risk of paravalvular leaks, thrombosis, and structural deterioration.
Enhanced device design allows for safer implantation, compatibility with minimally invasive techniques, and improved long-term performance. Patients benefit from reduced procedural complications, faster recovery, and better quality of life, supporting market growth and adoption rates.
Post-Operative Care and Recovery
Surgical outcomes are influenced by post-operative care, including monitoring, medication adherence, and rehabilitation. Effective post-procedural management reduces complications such as valve thrombosis, heart failure, or infection.
Remote monitoring technologies and digital health solutions enhance patient follow-up, ensuring timely detection of complications and supporting recovery. Hospitals that integrate post-operative care protocols improve overall outcomes, reinforcing confidence in heart valve devices.
Comparative Analysis of Outcomes
Comparing outcomes between device types and procedure methods helps identify optimal treatment strategies. Studies indicate that minimally invasive procedures combined with advanced transcatheter valves yield faster recovery but may have higher initial costs compared to conventional surgery.
Mechanical valves offer long-term durability with higher anticoagulation risk, while bioprosthetic valves reduce medication dependence but require re-intervention over time. These insights guide healthcare providers in selecting the most suitable approach for individual patients, ensuring better outcomes and market adoption.
Regional Differences in Surgical Practices
Surgical outcomes vary by region due to differences in healthcare infrastructure, training, and access to advanced devices. North America and Europe benefit from widespread availability of both conventional and minimally invasive procedures, with established post-operative care systems.
Asia-Pacific and emerging markets are expanding access to advanced devices and minimally invasive procedures through training programs, hospital investments, and regulatory support. Improved regional practices enhance patient outcomes and encourage adoption of heart valve devices globally.
Future Outlook: Improving Outcomes Through Innovation
The heart valve devices market will continue evolving as innovation enhances surgical outcomes. Personalized devices, advanced imaging, and minimally invasive technologies will reduce complications, improve precision, and shorten recovery times.
Integration of digital health tools and remote monitoring will support post-operative care and continuous patient evaluation, further improving procedural success. As outcomes improve, healthcare providers and patients are more likely to adopt advanced heart valve devices, driving market growth worldwide.
Conclusion
Surgical outcomes in the heart valve devices market are determined by device type, procedure method, surgeon expertise, and post-operative care. Advanced valve designs, minimally invasive techniques, and effective monitoring improve recovery, patient safety, and long-term device performance. By analyzing and optimizing these factors, healthcare providers can enhance outcomes, encourage adoption, and support sustained growth in the heart valve devices market globally.