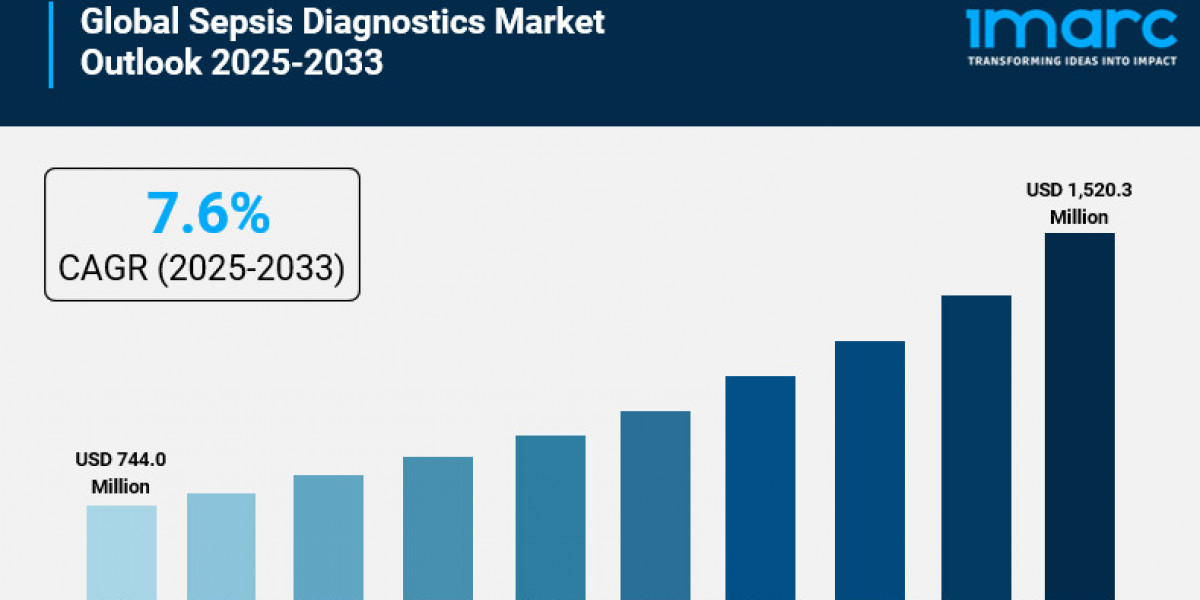
The global sepsis diagnostics market report was valued at USD 744.0 Million in 2024 and is projected to reach USD 1,520.3 Million by 2033. The market is expected to grow at a compound annual growth rate (CAGR) of 7.6% during the forecast period of 2025 to 2033. This growth is driven by factors such as increasing infectious disease incidences, expanding healthcare infrastructure, technological advancements, and growing adoption of data analytics in diagnostics.
Study Assumption Years
- Base Year: 2024
- Historical Year/Period: 2019-2024
- Forecast Year/Period: 2025-2033
Sepsis Diagnostics Market Key Takeaways
- Current Market Size: USD 744.0 Million in 2024
- CAGR: 7.6% during 2025-2033
- Forecast Period: 2025 to 2033
- The rising prevalence of sepsis due to aging populations and increasing chronic diseases is a major market driver.
- Automation and AI technologies are increasingly used to improve diagnostic accuracy.
- Point-of-care testing adoption is growing rapidly in critical care and emergency settings.
- North America leads the market, powered by advanced healthcare infrastructure and regulatory support.
- Technological innovations like molecular diagnostics and biomarker-based tests are enhancing diagnostic capabilities.
Get your Sample of Market Insights for Free: https://www.imarcgroup.com/sepsis-diagnostics-market/requestsample
Market Growth Factors
The worldwide sepsis diagnostics market evolution is mainly influenced by the increased sepsis prevalence, especially among the elderly and those with long-term diseases. The sepsis instances are going up all over the world, thus the need for sepsis diagnostic tools for the management and treatment of the condition is also increasing at a fast rate. Additionally, the market is getting a lift from constant advancements in molecular diagnostics and the introduction of biomarker-based testing that make early and more accurate detection of sepsis and related infections possible.
Among the propelling factors to the market are the innovations in technology such as the use of automation, artificial intelligence, and machine learning. These technologies lead to higher diagnostic accuracy and a shorter time to get the results, thus the initiation of the treatment can be done even faster. Moreover, the downsizing of diagnostic instruments and the process of automation have made it possible to carry out point-of-care testing in intensive care units and emergency departments in a better way, thus the rapid decision-making of a critically ill patient is facilitated.
One of the important market drivers is the efforts of governments and the public health policies to create awareness and improve the sepsis detection. Enhanced funding in research and development as well as educational programs for healthcare professionals will greatly promote the use of advanced diagnostic technologies. Besides, the regulatory approvals of the newly developed diagnostic devices such as the tests that directly detect bacteria in the blood samples without the need for culture will make the market grow faster and be adopted more widely in different parts of the world.
Market Segmentation
- Diagnosis:
- Automated Diagnostics: Use of advanced automated systems to expedite and streamline sepsis diagnosis, reducing turnaround time for results and enabling earlier treatment.
- Conventional Diagnostics: Traditional laboratory tests and clinical assessments manually interpreted by healthcare professionals to identify infection and immune responses.
- Product:
- Instruments: Essential devices used in sepsis diagnostics workflows to conduct various tests.
- Assay Kits and Reagents: Chemical and biological substances necessary for diagnostic procedures.
- Blood Culture Media: Medium supporting the growth and identification of pathogens in blood samples.
- Software: Programs facilitating data analysis and management in diagnostic workflows.
- Mode of Testing:
- Laboratory Tests: Comprehensive diagnostics requiring specialized equipment and trained personnel.
- Point-of-Care Tests: Rapid bedside tests enabling quick diagnosis and treatment initiation.
- Technology:
- Molecular Diagnostics: Techniques like PCR and nucleic acid sequencing to rapidly identify pathogen genetic material.
- Immunoassays: Tests such as ELISA detecting sepsis-associated biomarkers like procalcitonin and C-reactive protein.
- Biomarkers: Molecules like PCT, CRP, and interleukins used for diagnosing and monitoring sepsis.
- Flow Cytometry: Analyzes immune response by characterizing immune cells in blood.
- Microfluidics: Devices allowing multiple tests on minimal sample volumes simultaneously.
- Blood Culture: Method to identify causative organisms by growing them from blood samples.
- Pathogen:
- Bacterial Sepsis:
- Gram-Positive Bacterial Sepsis: Caused by bacteria such as Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus faecalis.
- Gram-Negative Bacterial Sepsis: Caused by bacteria like Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa.
- Viral Sepsis: Triggered by viral infections, including influenza, herpes simplex virus, and HIV.
- Fungal Sepsis: Due to fungal species such as Candida and Aspergillus.
- Others: Other pathogens causing sepsis.
- End User:
- Diagnostic Clinics: Facilities offering rapid tests like blood gas analysis and biomarker assays for quick results.
- Hospitals: Provide comprehensive services including complete blood counts, blood cultures, imaging, and coagulation studies.
- Research Laboratories: Focus on identifying and characterizing novel biomarkers for sepsis diagnostics.
Regional Insights
Sepsis diagnostics market in North America is the leading region due to the advanced healthcare infrastructure and a regulatory environment. In the U.S., annually, around 1.7 million adults suffer from sepsis of which an estimated number of 270,000 result in death. Canada reports 40,000 to 50,000 sepsis cases yearly and the disease ranks as the 12th leading cause of death. Furthermore, the Canadian Sepsis Foundation is estimating the cost of sepsis treatment at US$ 325 Million per year. All these factors are the reasons behind the high market demand in the area.
Recent Developments & News
- November 2023: Inflammatix, Inc. completed technical development of its TriVerity Acute Infection and Sepsis Test System, intended for emergency departments to assess bacterial and viral infections and ICU risk.
- August 2023: The CDC launched the Hospital Sepsis Program Core Elements to help U.S. hospitals establish effective sepsis identification teams and improve patient survival.
- May 2022: Sepsis Alliance initiated the Sepsis Innovation Collaborative (SIC) to enhance early diagnosis and develop improved antimicrobial therapies; members include Beckman Coulter, Merck, BioAegis Therapeutics, and Roche.
Key Players
- Abbott Laboratories
- Becton, Dickinson Company (BD)
- Biomerieux SA
- Bruker Corporation
- EKF Diagnostics Holding Plc
- Epic Systems Corporation
- Luminex Corporation
- Roche Holding AG
- Sysmex Corporation
- T2 Biosystems Inc.
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
Ask Our Expert & Browse Full Report with TOC & List of Figures: https://www.imarcgroup.com/request?type=report&id=3090&flag=E
About Us
IMARC Group is a global management consulting firm that helps the world’s most ambitious changemakers to create a lasting impact. The company provide a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us
IMARC Group,
134 N 4th St. Brooklyn, NY 11249, USA,
Email: sales@imarcgroup.com,
Tel No: (D) +91 120 433 0800,
United States: +1-201971-6302