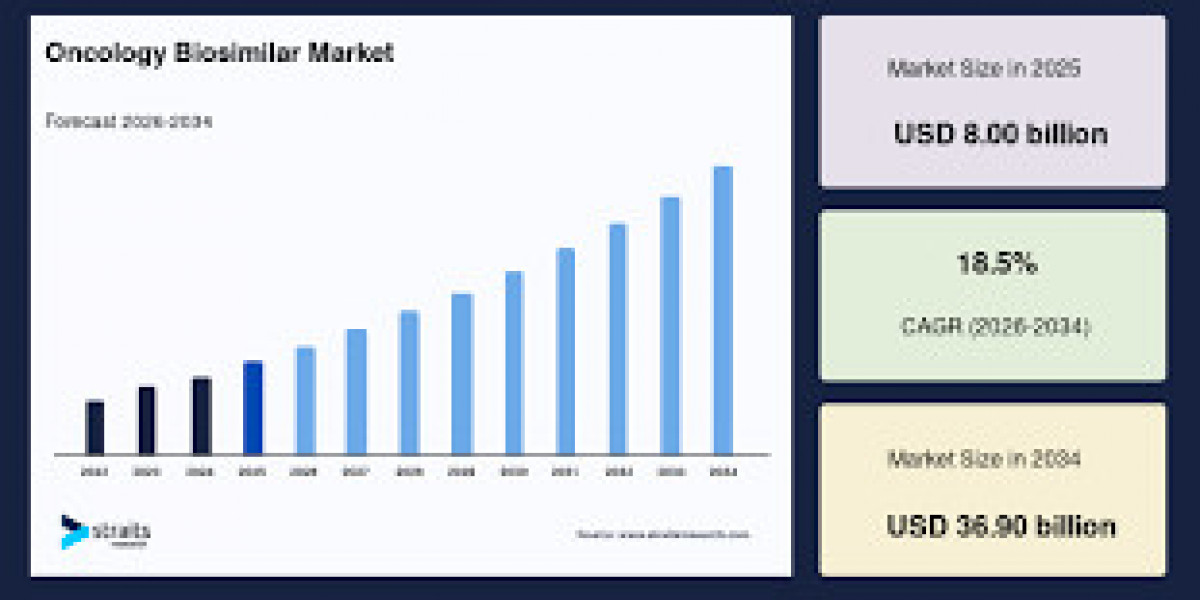
The global Oncology Biosimilar Market is projected to witness robust growth through the forecast period, expanding from an estimated USD 9.48 billion in 2026 to USD 36.90 billion by 2034 at a CAGR of 18.5%. The growth is driven by rising cancer incidence, patent expiries of blockbuster biologics, and increasing adoption of cost-effective biosimilar therapies worldwide.
Key Highlights
Largest Region: North America held the largest share of the oncology biosimilar market in 2025, accounting for approximately 38.14% of total revenue, supported by strong healthcare infrastructure and favorable regulatory policies.
Fastest-Growing Region: Asia Pacific is forecast to be the fastest-growing region, exhibiting a notable CAGR of 19.8%, fueled by expanding healthcare access, growing cancer burden, and local manufacturing growth.
Largest Segment – Product Type: Granulocyte Colony-Stimulating Factor (G-CSF) biosimilars held the largest share in 2025 with approximately 35% market revenue.
Fastest-Growing Distribution Channel: Hospital pharmacies are emerging as a high-growth channel, with a forecasted CAGR of over 18%, driven by centralized procurement and infusion-based oncology care.
Market Dynamics
Drivers:
The oncology biosimilar market is experiencing accelerated adoption due to significant cost advantages over reference biologics, leading to greater healthcare savings and expanded patient access. Biosimilars such as trastuzumab, rituximab, and bevacizumab offer 30–80% cost savings compared to originator drugs, enabling healthcare systems and payers to reallocate resources toward advanced oncology treatments. Regulatory frameworks like the U.S. Biologics Price Competition and Innovation Act and streamlined biosimilar approval pathways have further bolstered market uptake.
Restraints & Opportunities:
Despite growth momentum, market expansion faces headwinds from complex patent litigation and data exclusivity barriers that delay biosimilar launches and limit competitive penetration. However, strategic partnerships, co-development agreements, and portfolio expansion initiatives are creating lucrative opportunities especially in high-value therapeutic segments like immuno-oncology biosimilars. These collaborations are expected to unlock new product pipelines and broaden market reach in emerging and established regions alike.
Explore Market Trends and Forecasts - Download Your Free Sample:
https://straitsresearch.com/report/oncology-biosimilar-market/request-sample
Top Market Players
The global oncology biosimilar landscape features prominent pharmaceutical and biotech firms, including:
Pfizer Inc.
Amgen Inc.
Novartis International AG
Biocon Ltd.
Dr. Reddy’s Laboratories
Samsung Bioepis
Mylan N.V. (now part of Viatris)
Allergan (AbbVie)
STADA Arzneimittel AG
Apotex Inc.
Roche (via biosimilar affiliates)
Sandoz (Novartis division)
Celltrion Inc.
Bio-Thera Solutions
Organon
Teva Pharmaceuticals
Market Segmentation
By Product Type:
Monoclonal Antibodies (mAbs)
Hematopoietic Agents
Granulocyte Colony-Stimulating Factor (G-CSF)
Immunomodulators
By Cancer Type:
Breast Cancer
Lung Cancer
Colorectal Cancer
Cervical Cancer
Blood Cancer / Hematological Cancers
Others
By Route of Administration:
Intravenous (IV)
Subcutaneous (SC)
Others
By Distribution Channel:
Hospital Pharmacies
Retail Pharmacies
Online Pharmacies
Others
By Region:
North America
Europe
Asia Pacific (APAC)
Middle East and Africa (MEA)
Latin America (LATAM)
About the Report
The Oncology Biosimilar Market report provides a comprehensive analysis of industry trends, growth drivers, market dynamics, competitive landscape, and future forecasts through 2034. It includes detailed segmentation by product type, cancer indication, administration route, distribution channel, and regional outlook, offering essential insights for stakeholders, investors, and industry decision-makers seeking strategic guidance in the expanding oncology biosimilar space.